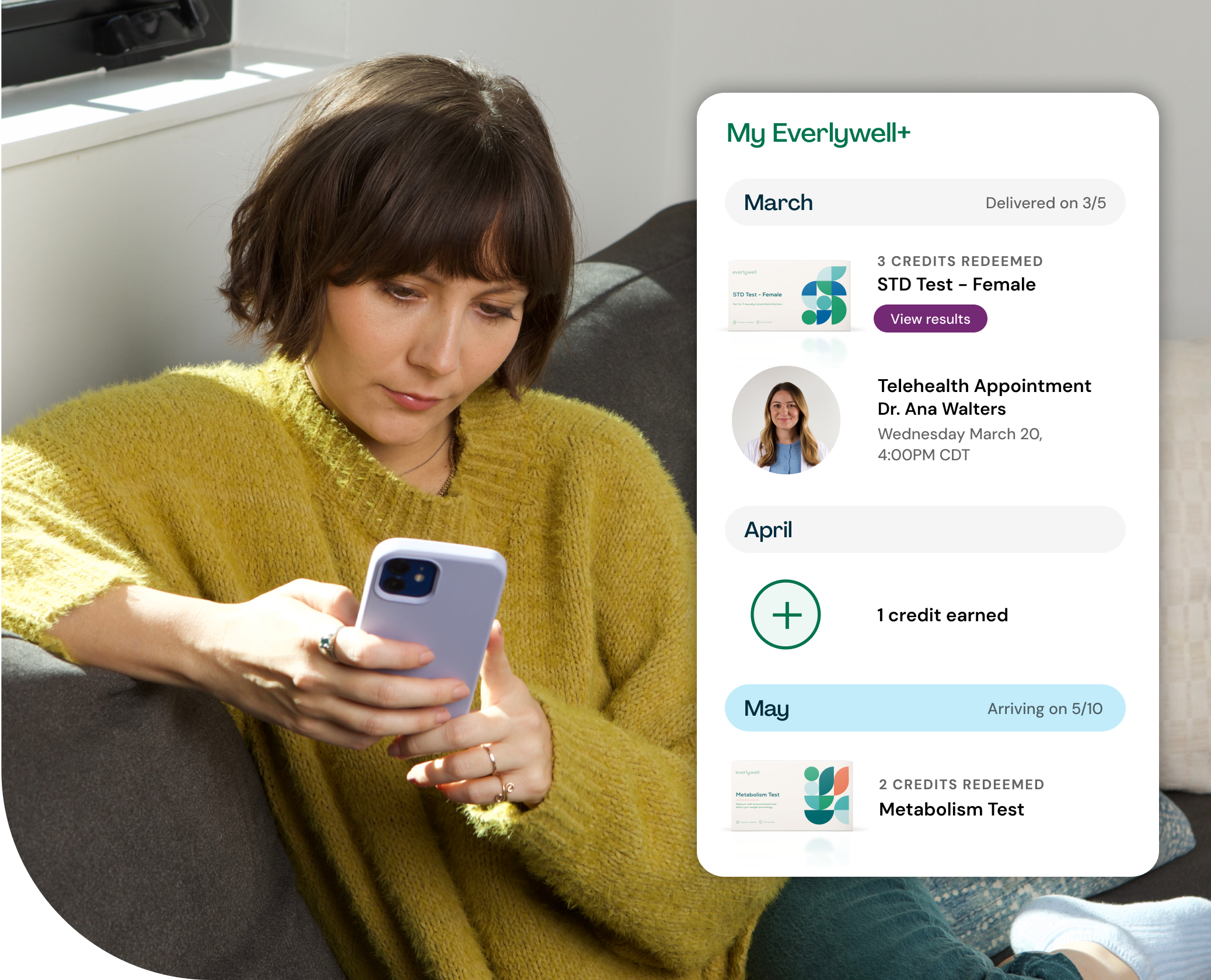
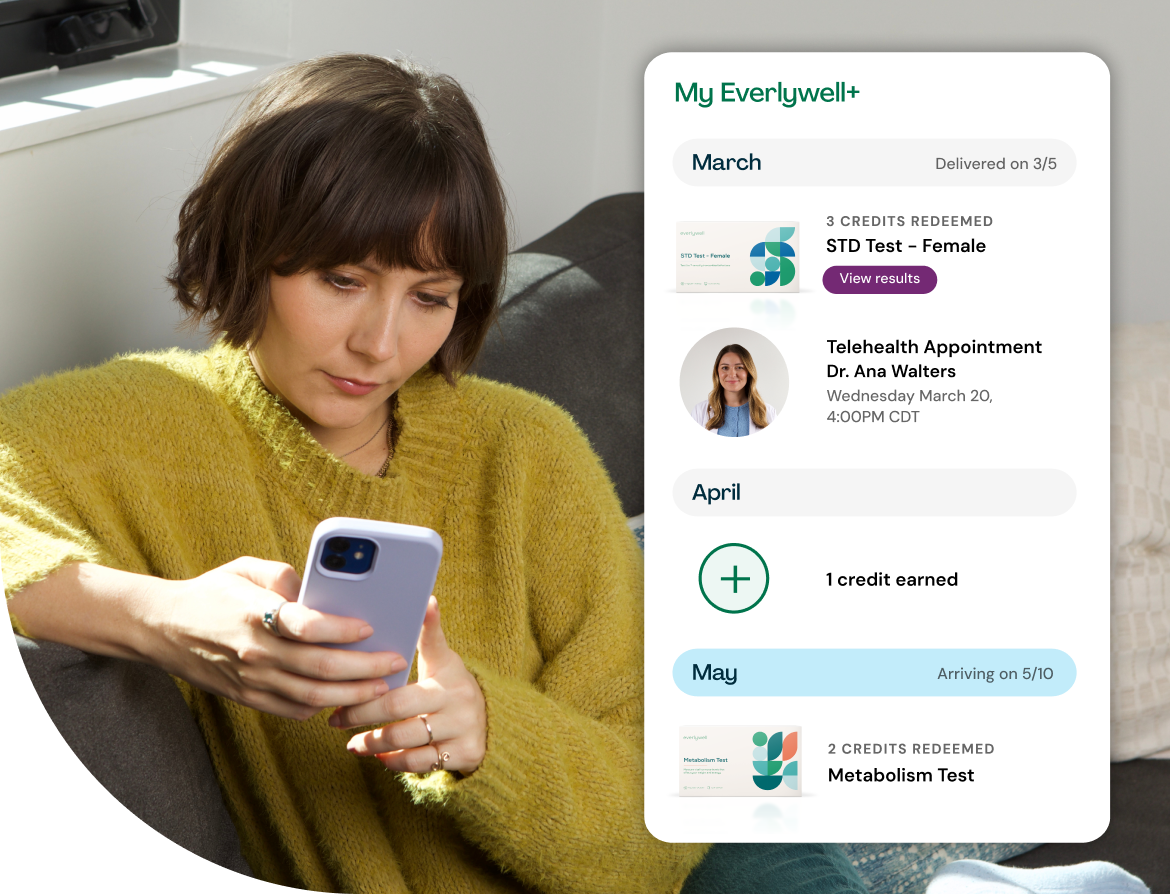
Introducing Everlywell+
Keep up with your wellness all year long as an Everlywell+ member. Earn a monthly credit toward your choice of any at-home lab test and get 20% off cash-pay telehealth and additional tests.

Help with weight management >

Plan for your health goals >

Support your energy levels >

Online treatment for COVID-19 >

Discreet STD care >

Check your thyroid health >

Wellness plans for women >

Fast UTI treatment >

Quick bacterial vaginosis treatment online >

Fast yeast infection treatment online >

Discreet cold sore treatment online >
Convenient online genital herpes treatment >
You’ve got symptoms. We’ve got treatments.
Schedule virtual visit




